In the field of renewable energy, hydrogen is considered to be one of the hopes for utilizing sources of abundant energy where they are most urgently needed. Surplus sunlight or wind can be used to create H2 in order to power trains, power ships or feed electricity into the grid for when it is dark and windless. However, the current process of hydrolysis, i.e., the splitting of water by means of an applied voltage, requires complex equipment. In addition, the resulting efficiency is substandard because the electricity is first generated by photovoltaics and then used for hydrogen production.
The example of photosynthesis shows that sunlight alone is completely adequate for the breaking down and reassembling of molecules. This requires functional molecules or nanoparticles and a structure of polymers that ensure the correct arrangement of the individual components.
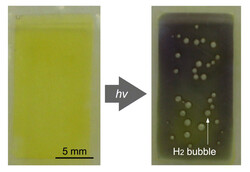
Researchers in Japan have succeeded in replicating the principle of photosynthesis in such a way that CO2 is not converted into sugar, but water is instead broken down into its basic components. Ruthenium and platinum, which are also used in vehicle catalytic converters and fuel cells, are used for this. Hydrogen can then be generated directly from the water via solar radiation.
What makes this process particularly remarkable is the efficiency, which is currently said to be in the range of photovoltaics, i.e., around 20%. This aspect is particularly important so that hydrogen can be produced sensibly and cost-effectively. Plants, on the other hand, only manage to convert 1% of the energy obtained from sunlight under normal conditions.
There are still a few hurdles to overcome, however. Large-scale utilization of the technology has not yet been tested, and the durability of the so-called hydrogel also still has to be proven. And last but not least, platinum and ruthenium are not exactly common elements. The latter is one of the rarest elements of all.
Not ideal conditions, but a few uncertainties should not deter researchers from the idea of producing hydrogen using sunlight alone.