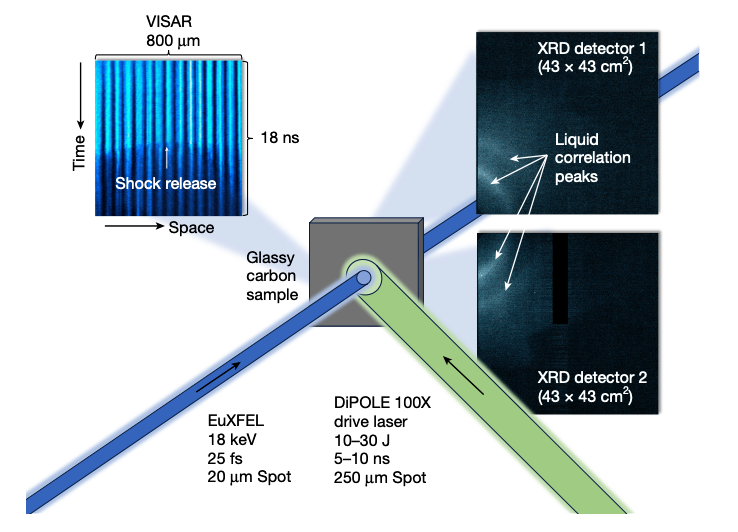
Researchers have captured the first detailed X-ray diffraction snapshots of liquid carbon under pressures near one million atmospheres, revealing a transient, tetrahedrally-bonded fluid rather than a densely packed atomic soup. The measurements, made by firing the DiPOLE 100-X laser into glassy carbon and probing the shocked matter with 18 keV pulses from the European XFEL, show about four nearest neighbors per atom—far from the dozen expected in simple liquids—and provide a solid benchmark for quantum-molecular-dynamics simulations of carbon at extreme conditions.
The team observed the transition from amorphous carbon to diamond at ~80 GPa, followed by complete melting into the liquid at ~160 GPa. Fourier analysis of the diffraction data indicated a first-shell coordination number of 3.78 ± 0.15 and a modest 7 percent volume jump at melt, values consistent with recent first-principles calculations. These data also allowed an experimental estimate of the latent heat of fusion (~130 kJ mol-1) and validated the positive 11 K GPa-1 slope of carbon’s melt curve in this pressure range.
Such microscopic insight matters for inertial-confinement fusion (ICF). Present ignition designs, including the National Ignition Facility’s record-setting shot, rely on a high-density carbon (diamond) shell that surrounds and symmetrically compresses a deuterium-tritium target. That shell is intentionally driven close to its melting point during the initial shock; its response—strength, opacity, heat capacity—sets the stage for the rest of the implosion. A complete picture of liquid carbon’s structure and equation of state, therefore, feeds directly into future fusion ablator design and predictive hydrodynamic modeling.
The study also highlights the performance gap between crystalline and amorphous carbon coatings. Emerging ICF concepts explore lower-density, hydrogen-rich amorphous films to mitigate pre-heat and improve implosion symmetry. The new liquid-state data offer a route to tailor those films: matching porosity, tuning optical depth, and selecting compositions that maintain favorable melt characteristics under shock loading.
Beyond direct target fabrication, the results act as a high-quality training set for machine-learning interatomic potentials, which dramatically speed up molecular-dynamics simulations of carbon under shock—letting you reach larger system sizes and longer timescales than would otherwise be practical.
Source(s)
Nature (in English)